Farmers and farm managers across California face many challenges while making every drop of water count. Proper irrigation system maintenance is essential for maximizing efficiency, reducing costs, and ensuring healthy crops and high yields. One often overlooked but critical maintenance practice is dripline flushing.
Regularly flushing your irrigation system helps prevent debris buildup, maintains consistent system pressure, and minimizes the risk of clogged systems—all of which contribute to better distribution uniformity and improved crop performance. By integrating dripline flushing into your maintenance routine, you can enhance system efficiency, reduce troubleshooting costs, and ultimately boost yields. This article will explore how and when to flush your system for optimal results.
Why Dripline Maintenance Matters
- Flushing the irrigation system reduces the accumulation of debris to a minimum by flushing the system.
- Systems should be flushed at regular intervals. The frequency depends on your water quality and maintenance program.
- There are usually two waves of flushed contaminants: 1) the first wave removes contaminants collected at the end of the dripline; 2) the second wave removes contaminants from the rest of the dripline.
The Benefits of Dripline Flushing
- Consistent system pressure, flow rate, and distribution uniformity
- Improved system efficiency and lower costs
- Minimize water and pumping costs
- Minimize costs related to troubleshooting dripline plugging and distribution issues
- Improved crop health and yields
The Steps for Dripline Flushing
- Pressurize the system
- Go to the dripline ends
- Open the dripline’s end to flush (one at a time, or more depending on the system)
- Wait until the water comes out clean and freely
- Close the dripline’s end (one at a time, or more depending on the system)
- Repeat the flushing process with all dripline ends
- Go to the sub-main flushing valve
- Open the sub-main flushing valve
- Wait until the water comes out clean and freely
- Close the sub-main flushing valve
Drip System Maintenance and Troubleshooting Problems
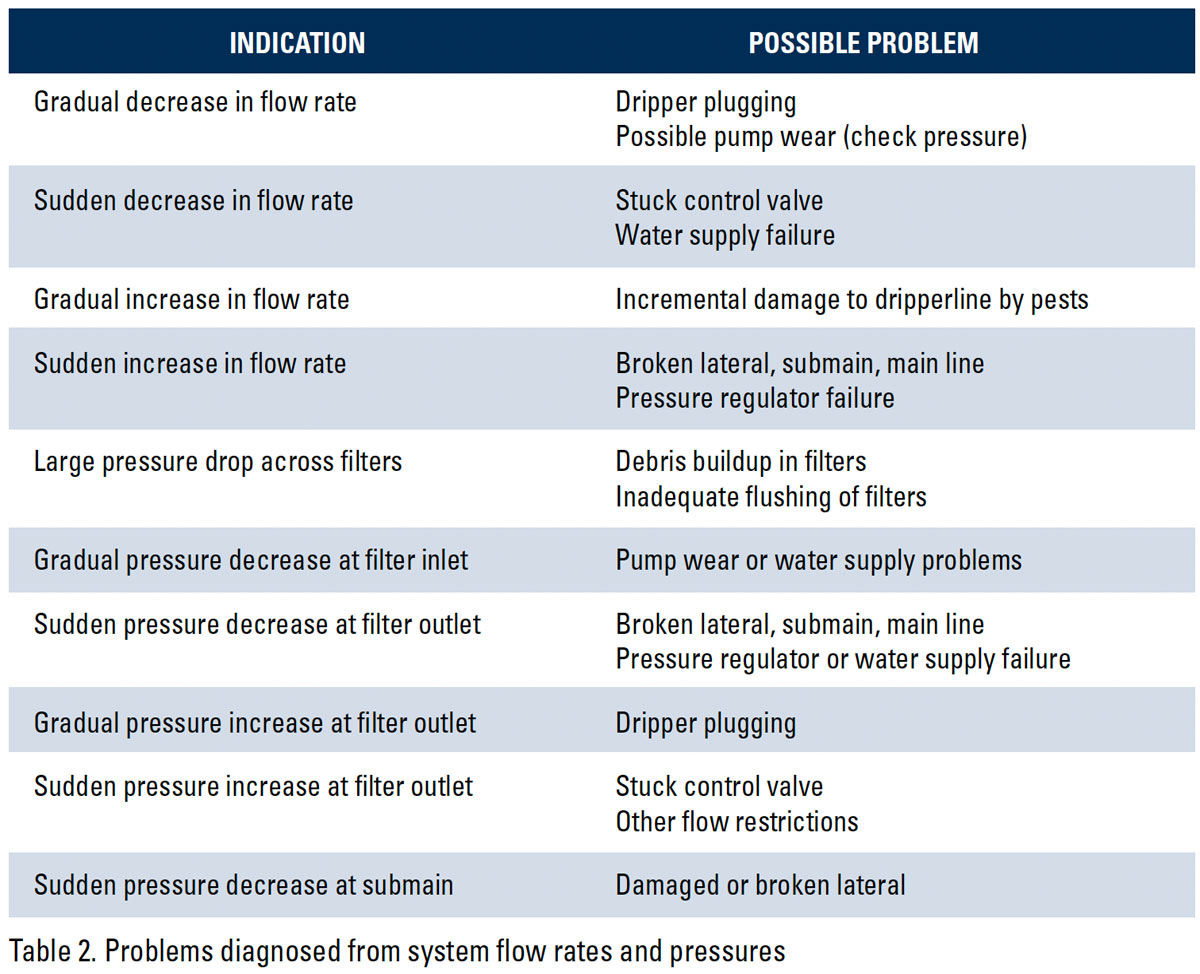
The maintenance of your SDI system centers on the identification of the factors that can lead to performance reductions of your drip system and procedures to mitigate these negative impacts. Factors that can slow or stop flow through the drip system include: suspended material, chemical precipitation, biological growth, root intrusion, soil ingestion, and crimping of the driplines.
Ensure maximum system life by reducing or eliminating the impact of negative factors (Table 2). This may require water treatment and a systematic program for regular maintenance. In this section, we outline the various potential issues that can adversely affect the drip system and offer procedures to mitigate the potential damage.
Water Quality
The potential for dripper plugging problems will vary with the source of the irrigation water, either surface or groundwater. In general, algae and bacterial growth are usually associated with the use of surface water. Whole algae cells and organic residues of algae are often small enough to pass through the filters of an irrigation system. These algae cells can then form aggregates that plug the drippers. Residues of decomposing algae can accumulate in pipes and drippers to support the growth of slime-forming bacteria. Surface water can also contain larger organisms such as moss, fish, snail, seeds, and other organic debris that must be adequately filtered to avoid plugging problems. Groundwater, on the other hand, may contain high levels of minerals that can challenge dripper function. Water from shallow wells (less than 100 feet) often will produce plugging problems associated with bacteria. Chemical precipitation is more common with deep wells.
A water quality analysis can give the grower a “heads-up” on potential trouble areas for the drip system. This test should be accomplished before the final design of the system to ensure that proper components are installed to address any problem areas. Many laboratories around the United States have Water Quality Analysis services available that can conduct a “Drip Irrigation Suitability Test.” The analysis should include testing for pH, dissolved solids, manganese, iron, hydrogen sulfide, carbonate, and bicarbonates. Table 3 lists the more common water quality issues that can affect drip irrigation systems. Having a water analysis in the moderate or even severe category does not mean drip irrigation cannot be used, but only special precautions must be applied to prevent problems. Consult your local Netafim USA Dealer for more information on water quality and drip irrigation.
Suspended Solids
Suspended solids in the incoming water are the most common stress impinging upon the drip system and the easiest to control. Each and every Netafim dripper has a large filter built into the unit to keep suspended particles from being trapped in the labyrinth. This filter is located at the bottom of the dripper and points toward the center of the drip tubing so that it can be cleaned by flushing the dripline. This built-in filter plays an important role in the longevity of the system. Thus, most water used for drip irrigation must be filtered to remove suspended solid particles that can lodge in the drippers and reduce or even stop the flow. These particles can be either organic such as algae or inorganic, such as sand. Each manufacturer recommends a filtration level based on the technology of the dripper device. The Netafim drippers commonly require 120 mesh filtration. This is the lowest filtration requirement of any commercial drip irrigation product. That means that the drippers are more reliable, ensuring long service even under harsh conditions.
Surface water generally contains a combination of organic and inorganic suspended particles. These include algae, moss, and aquatic animals, as well as suspended sand, silt, and clay particles. Filtering this mix of material is a challenge that is best accomplished using three-dimensional filtration, such as sand media or disc. Well water generally has lower levels of suspended solids which can be handled using disc filtration or, in cases of very low contaminant levels, screen filters. If large quantities of sand are being generated by the well, a sand separator may be installed before other filters. Filters should automatically self-clean (backflush) during operation when the contaminant levels get high enough.
Chemical Precipitation
Chemical plugging usually results from the precipitation of one or more of the following minerals: calcium, magnesium, iron, or manganese. The minerals precipitate from the solution and form encrustations (scale) that may partially or completely block the flow of water through the dripper. Water containing significant amounts of these minerals and having a pH greater than seven has the potential to plug drippers. Particularly common is the precipitation of calcium carbonates, which is temperature and pH-dependent. An increase in either pH or temperature reduces the solubility of calcium in water and results in the precipitation of the mineral.
When groundwater is pumped to the surface and discharged through a micro-irrigation system, the temperature, pressure, and pH of the water often change. This can result in the precipitation of calcium carbonates or other minerals to form scale on the inside surfaces of the irrigation system components. A simple test for identifying calcium scale is to dissolve it with vinegar. Carbonate minerals dissolve and release carbon dioxide gas with a fizzing, hissing effervescence.
Iron is another potential source of mineral deposits that can plug drippers. Iron is encountered in practically all soils in the form of oxides, and it is often dissolved in groundwater as ferrous bicarbonate. When exposed to air, soluble ferrous bicarbonate oxidizes to the insoluble or colloidal ferric hydroids and precipitates. The result is commonly referred to as ‘red water,’ which is sometimes encountered in farm irrigation wells. Manganese will sometimes accompany iron, but usually in lower concentrations.
Hydrogen sulfide is present in many wells. Precipitation problems will generally not occur when hard water, which contains large amounts of hydrogen sulfide, is used. Hydrogen sulfide will minimize the precipitation of calcium carbonate (CaC03) because of its acidity.
Fertilizers injected into a drip system may contribute to plugging. This may be the result of a chemical reaction that occurs when different fertilizers are mixed or because the fertilizer in question is not completely soluble. This type of plugging is completely preventable. To determine the potential for plugging problems from fertilizer injection, the following test can be performed:
- Add drops of the liquid fertilizer to a sample of the irrigation water so that the concentration is equivalent to the diluted fertilizer that would be flowing in the lateral lines.
- Cover and place the mixture in a dark environment for 12 hours.
- Direct a light beam at the bottom of the sample container to determine if precipitates have formed. If no apparent precipitation has occurred, the fertilizer source will normally be safe for that specific water source.
Biological Growth
A micro-irrigation system can provide a favorable environment for bacterial growth, resulting in slime buildup. This slime can combine with mineral particles in the water and form aggregates large enough to plug drippers. Certain bacteria can cause enough precipitation of manganese, sulfur, and iron compounds to cause dripper plugging. In addition, algae can be transported into the irrigation system from the water source and create conditions that may promote the formation of aggregates.
Dripper plugging problems are common when using water that has high biological activity and high levels of iron and hydrogen sulfide. Soluble ferrous iron is a primary energy source for certain iron-precipitating bacteria. These bacteria can attach to surfaces and oxidize ferrous iron to its insoluble ferric iron form. In this process, the bacteria create a slime that can form aggregates called ochre, which may combine with other materials in the micro-irrigation tubing and cause dripper plugging. Ochre deposits and associated slimes are usually red, yellow, or tan.
Sulfur slime is a yellow-to-white stringy deposit formed by the oxidation of hydrogen sulfide. Hydrogen sulfide (H2S) accumulation in groundwater is a process typically associated with reduced conditions in anaerobic environments. Sulfide production is common in lakes and marine sediments, flooded soils, and ditches; it can be recognized by the rotten egg odor. Sulfur slime is produced by certain filamentous bacteria that can oxidize hydrogen sulfide and produce insoluble elemental sulfur.
The sulfur bacteria problem can be minimized if there is no air-water contact until water is discharged from the system. Defective valves or pipe fittings on the suction side of the irrigation pump are common causes of sulfur bacteria problems. If a pressure tank is used, the air-water contact in the pressure tank can lead to bacterial growth in the tank, clogging the dripper. The use of an air bladder or diaphragm to separate the air from the water should minimize this problem.
Root Intrusion
Plant roots tend to grow toward soil areas with the highest water content. Because of this tendency, roots can clog subsurface drip systems by growing into the dripper openings. Plant roots tend to “hunt” for water when it is in short supply thus, the problem seems to be more acute when irrigation is not sufficient for the plant needs. This is a particular problem in systems that are left unused for part of the season. Several strategies can be employed to reduce the possibility of root intrusion:
- Short, frequent irrigations keep adequate water in the root zone so the roots have no need to look for water.
- Acid injection that lowers the pH to less than four will discourage root growth and can be used to clean roots out of drippers with small amounts of root intrusion. High concentrations of chlorine (100 to 400 ppm), N-pHURIC, phosphoric, or metam sodium (Vapam) will also destroy roots in the drippers.
- In areas where it is allowed, trifluralin is an effective inhibitor of root growth and can be used to prevent root intrusion.
- A seamed dripline encourages roots to grow along the seam and into the dripper. Netafim products are designed without a seam to discourage this intrusion.
Soil Ingestion
Soil ingestion is not a problem in properly designed drip systems. Soil injection occurs when soil is sucked into the dripline. When a drip system is shut off, the water continues to flow to the low end of the field, creating a vacuum at the higher end, sucking saturated soil into the line. A properly designed drip system will minimize this potential problem. The supply manifold must be equipped with vacuum relief vents, these vents allow air to flow into the driplines when the system is shut off. Netafim air/vacuum relief vents will allow sufficient air into the system. Insufficient air will create a vacuum (similar to not using vents). This is not a good place to skimp.
Other Maintenance Procedures
Filter Maintenance
Follow the standard instructions for the maintenance of your filter system. Filters are the first line of protection for your drip system and they need regular maintenance to operate at a high level. On a bi-weekly basis, observe the system as it completes a backflush cycle. Make sure all pressures are within the system limits before and after backflushing. Check the operation of backflush valves, pressure differential switches, and controllers. At the end of the season, check the media level in media tanks. Scum can build up on disc filters and the discs may need to be cleaned with acid. In areas that experience freezing temperatures, drain all water from the filter, valves, and control system.
Dripline Flushing
To minimize sediment build-up, regular flushing of drip irrigation pipelines is recommended. The system design should be such that a minimum flush rate of 1.0 ft/sec can be obtained in the lines. Valves large enough to allow sufficient flow velocity should be installed at the ends of mains, submains, and manifolds. Also, allowances for flushing should be made at the ends of lateral lines. Flushing of the drip lateral lines should continue until clean water runs from the flushed line for at least two minutes. A regular inspection and flushing maintenance program will help significantly prevent dripper plugging.
Chemical Treatment
Chemical treatment is often required to prevent dripper plugging due to microbial growth and/or mineral precipitation. The attachment of inorganic particles to microbial slime is a significant source of dripper plugging. Chlorination is an effective measure against microbial activity. Use chlorine and all other chemicals only according to label directions. Acid injection can remove scale deposits, reduce or eliminate mineral precipitation, and create an environment unsuitable for microbial growth.
Need Further Assistance with Drip System Repair and Maintenance?
As a full-service pump and irrigation dealer serving Central California, Cal-West Rain has over 35 years of experience working with growers, farm managers, and ranchers. As a California-licensed contractor, we provide turnkey pump and irrigation solutions. From new system design to construction, pumps, filtration, electrical, maintenance, and repairs. Contact us in Kerman, Hanford, Bakersfield or Paso Robles to discuss your project.
References & Resources
- How to Flush an Irrigation System | Netafim (video)
- Netafim Precision Irrigation Academy (Video resource library)
- Netafim Drip System Operation and Maintenance (EN) | (PDF)
- Netafim Operación y Mantenimiento Del Sistema De Goteo (ES) | (PDF)

